What is pharmaceutical manufacturing?
Pharmaceutical manufacturing, which is a component of the pharmaceutical industry, is the process of producing pharmaceutical medications on an industrial scale. A number of unit operations, including milling, granulation, coating, tablet pressing, and others, can be used to breakdown the drug production process into its component parts.
Importance of pharmaceutical drug manufacturing:
The pharmaceutical sector has ensured efficient drug production that aids in patient therapy, treatment, and life-prevention. The pharmacy industry has developed in providing medications & treatments that enhance quality of life in response to the rising number of health conditions on a global scale. Because of this, pharma has become incredibly important to human society.
- The final mixture used to make the solid dosage form during pharmaceutical manufacture may contain a variety of non-active substances in addition to the active pharmaceutical ingredient. Numerous factors must be taken into consideration due to the variety of materials that might be blended. Included in this list are the particle size distribution, particle shape , moisture content, particle surface characteristics including roughness and cohesiveness, and powder flow characteristics.
- However, contagious illnesses like the corona virus pandemic can impede the operation, effectiveness, and growth of healthcare systems. As a result, society as a whole is impacted. When such happens, the pharmaceutical sector plays a critical role in supplying the necessary treatments and resources to either avoid or resolve the crisis.
- Numerous industrial facilities around the world are propelled by a large labor force, highly skilled technicians, and human capacity. This facilitation not only generates profitable revenue for the country but also makes a sizeable contribution to GDP. The nation’s labor efficiency, which produces goods & services worth billions, is what draws massive investments from multinational companies around the world.
Drug manufacturing steps:
- To lower the average particle size in a medication powder, milling is frequently required during the production process. This is due to a number of factors, including improved homogeneity and dosage uniformity as well as improved medicinal ingredient solubility. To increase the blends’ ability to be manufactured, repeated powder blending followed by milling occasionally takes place.
- Wet granulation and dry granulation are the two main forms of granulation. Granulation can be thought of as milling’s reverse. Granules are larger particles made up of several smaller ones linked together. Granulation is employed for several purposes. By forming a granule with all the elements present in the right proportions, it prevents the “remixing” of the mixture’s components. This enhances the flow characteristics of powders and increases the compaction properties for tablet creation.
- In pharmaceutical solid oral dosage manufacturing, hot melt extrusion is utilized to deliver medications with low solubility and bioavailability. Poorly soluble medications can be molecular dispersed in a polymer carrier by hot melt extrusion. The process involves mixing materials and forcing them through a tool die under pressure, heat, and agitation. Twin-screw high-shear extruders combine ingredients while also dispersing particles. The resulting particles can be mixed together, crushed, and added to tablets or capsules.
- Dry ice can be used in laboratories to cool medications to improve reaction selectivity, but doing so at an industrial scale is difficult. The expense of cooling a standard reactor to this temperature is costly, and as the temperature drops, the viscosity of the reagent can rise, making mixing challenging. This either results in a non-homogeneous reaction or adds the cost of swirling more vigorously and replacing components more frequently.
Validation and Optimization of Drug manufacturing process
When a pharmaceutical product is validated, it indicates that all critical steps in its development, production, and control are trustworthy, repeatable, and able to deliver the appropriate level of product quality when carried out in accordance with the rules for production and control.
Qualification of personnel, facilities, and equipment is a requirement for and an essential component of process validation. In other words, when equipment is qualified, measuring devices are calibrated, and processes are validated, it is necessary to examine technical and physical parameters as well as chemical, physical, and biological ones.
As is generally known, validation begins with the development of a new machine or piece of equipment, as well as a new or modified product.
This method of development, which starts with laboratory and clinical trials and continues through the production phase, must guarantee that items are manufactured according to the targeted profile.
GMP requirements must be followed when creating pharmaceutical items for clinical trials. For instance, the manufacturing facility where the drug product is created must be accredited, and the procedure must be confirmed. As a result, even when scaling up has not yet been finished, manufacturing processes for clinical trials should be validated at the conclusion of the development phase, as soon as processes are at least partially optimized.
Such a validations-report, which primarily seems to be an optimization-report, summarizes the findings of all development studies, including the use of equipment, the tolerances of physical parameters in the manufacturing process, and the accepted specification of beginning materials. When scaling up in the production plant is finished, this can be especially valuable.
This approach can be prospectively verified as long as there are enough clinical trial batches produced using an approved method. The validation of production conditions is based on the findings of this validation as well as the knowledge obtained during optimization.
Drug development involves :
Step 1: Discovery and Development:this includes the experimental studies designed to move a program from the initial identification of a biological target and associated disease state to the identification of single compound with the potential to be clinically relevant. It can also have different stages like target discovery, lead discovery, and lead optimization.
Step 2: Pre-clinical Research: Here scientists do their laboratory experiments then test their ideas on new drug sometimes they can do tests on animals.
Step 3: Clinical Research:Here scientists study how safe the drug is and treatments that can be used to prevent or diagnose a certain disease.
Step 4: FDA Drug Review:it takes 6-10 months to review if a drug can be consumed or used. Here the drug developer must provide the following information to FDA those information include
-proposed labeling
-drug abuse information
-patent information
-safety updates
-the study data if the drug has been conducted outside the united states -directions for use
After all these information each member of the review team will conduct the full review according to his/her section.Then FDA inspector conduct a routine investigation to see if there was no evidence of manipulation, fabrication or withholding data.
Step 5: FDA Post-Market Drug Safety Monitoring(after drug approval): After the FDA review in case it has been proved that the drug is safe and effective for its use they need to approve. So they need to monitor the safety of that drug(to see if it meets the agency(FDA) standards) before going to the market.
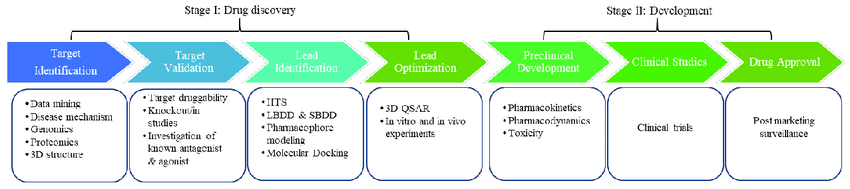
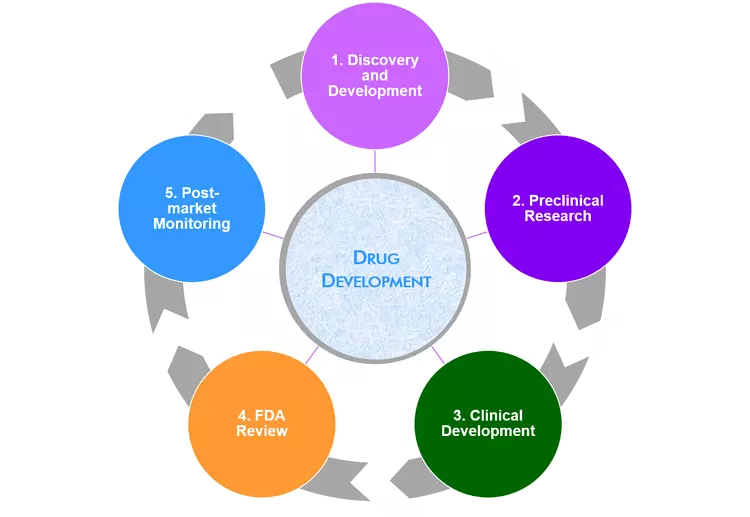
It take an average of 12 years of a drug to be on the market.
Why choosing senieer?
Leading global manufacturer and supplier of pharmaceutical equipment, Senieer offers ONE STOP SOLUTION. Senieer is the finest global partner for businesses in the pharmaceutical, food, chemicals, and cosmetics sectors. For more than 34 years, Senieer has focused mainly on solid dosage forms in China. Reliable integrated process solutions. We produce equipment in compliance with global norms including GMP, cGMP, and US FDA.
Members of the Senieer technical team enhance one-stop solution services for comprehensive projects, from consulting to design. We fully satisfy your method and requirements for resolving all of your issues.










