At present, with China’s GMP regulations in line with international standards, the level of drug production quality management has been greatly improved. However, it needs to be clearly recognized that in the process of implementing GMP, there are still many problems, such as the concept of quality management needs to be strengthened, the quality culture has not been formed, the technical aspects are still conformist, and the internal management is lack of effective inspection and supervision. This paper discusses the main problems that may be encountered in the implementation of GMP from four aspects: writing what you do, doing what you write, recording what you do and correcting your mistakes, and summarizes and puts forward corresponding improvement measures to promote the more effective implementation of GMP regulations in pharmaceutical enterprises.
Since 1999, it has been 22 years since the compulsory implementation of GMP in the field of drug production at the national level. It has been 10 years since the implementation of the new version of GMP (revised in 2010) on March 1, 2011. China’s GMP laws and regulations have been in line with the international standard EU GMP, which has greatly improved the level of drug production quality management in China. However, we need to clearly realize that there are still many problems in the implementation of GMP, such as the concept of quality management needs to be strengthened, the quality culture has not been formed, the technical aspects are still rigid, and the internal management is lack of effective inspection and supervision. This paper analyzes and discusses the main problems that may be encountered in the implementation of GMP, summarizes and puts forward corresponding improvement measures, so as to promote the more effective implementation of GMP regulations in pharmaceutical enterprises.
What Is GMP
The Chinese name of GMP is Quality Management Practice for Pharmaceutical Production, which is a part of the quality management system. The concept of GMP can be understood as: write what you do, do what you write, remember what you do, and correct what you are wrong (as shown in Figure 1). “Write what you do”, coordinate the layout and establish the implementation standard; “Do what you write”, emphasize the implementation of the standard according to the rules; “Remember what you do”, the data is complete for retrospective inspection; “Correct what you are wrong”, Summarize and improve, constantly revise and improve. The implementation of GMP is also a circular process from establishing standards, implementing standards, forming records, improving standards, and then establishing standards. In the process of practice, the level of quality management has been effectively improved.
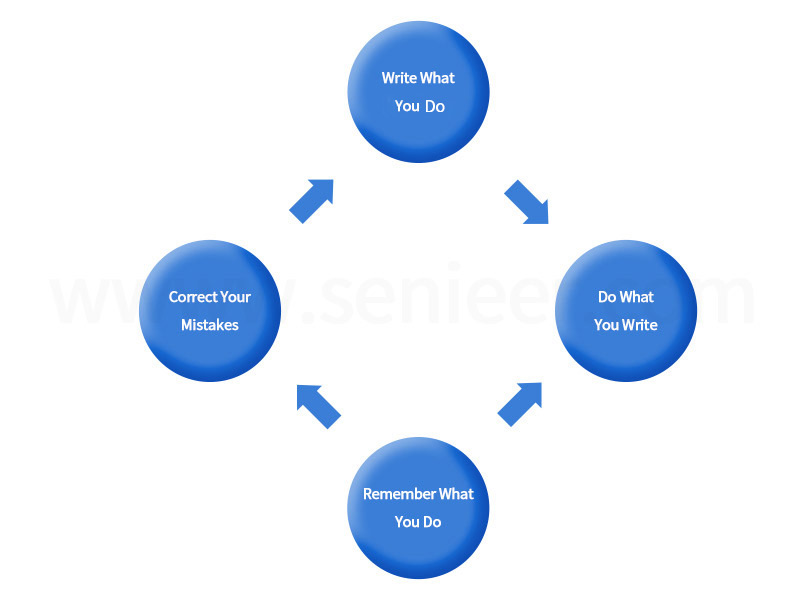
FIG. 1 Conceptual understanding of GMP
The Main Problems And Analysis Of The Implementation Of GMP
-
Write What You do
(1) The document system is plagiarized and applied without taking into account the actual situation of the enterprise and the latest regulatory requirements.
(2) When the actual operation of the post is inconsistent with the provisions of the document system, it is not revised in time.
(3) When the management personnel drafted and prepared the documents, the post operators did not participate in the preparation and revision of the documents, so that the provisions of the documents were out of touch with the actual situation.
(4) The document review is not strictly controlled, and some documents are not operable themselves.
Example Of GMP Inspection Defects: The company failed to carry out relevant training, document formulation and implementation in accordance with the requirements of computerized system verification and validation and verification appendix.
Chapter 8 of GMP (2010 Revised Edition) makes detailed provisions on document management. The compilation of the company’s GMP file system reflects the management’s awareness of legal compliance and the technical management level of the technical team. A good SOP file must meet the 5W1H requirements (as shown in Figure 2). Documents are a tool to ensure the effective transmission and management of information. From the high-level emphasis on providing resources, creating a culture of compliance and quality, and then unifying the thinking of all employees, combining actual discussions to reach a consensus, paying attention to regulations and standards to update the document system in a timely manner, and then mobilizing front-line employees to actively Participate in, constantly check and correct, carry out risk management on the system at the system level, and finally do a good job in the top-level design of the effective implementation of GMP.
-
Do What You Write
(1) No substantive training was carried out before the document was executed, or the training effect was not evaluated, and the practical ability was not confirmed.
(2) Do not pay attention to the documents during the post operation process, and the management personnel will direct and replace them on the spot.
(3) The documents required for the post are inconvenient to read or have not been issued.
(4) The post operator did not really understand the document and did not execute it one by one according to the requirements of the document.
Examples of GMP inspection defects: the approval, reproduction, distribution, and destruction of documents are not managed according to the procedures, there is no record of reproduction, distribution, replacement or destruction, the “Code List of Raw Materials Suppliers” has no document number, and the production has not been approved by the quality department. The department has provided two versions of “Process Specifications for Dibazol Tablets”, of which the old version (STP-GY-016-R00) has expired, and the current version (STP-GY-016-R01) is not under control.
Article 184 of GMP stipulates that the production and packaging of all medicines shall be operated in accordance with the approved technological procedures and operating procedures, and relevant records shall be kept [2]. Operating in accordance with the procedures is the basic quality and requirement of every employee. Managers should go deep into the production line to understand the implementation of the document system, regularly check the consistency of document regulations and implementation, and pay attention to the understanding of the content of documents by front-line employees. This is also an inspection and supervision of the effectiveness of the quality management system.
-
Remember What You Do
(1) The personnel did not form the habit of filling in the records in a timely, accurate and complete manner, and there was a problem with the integrity of the data.
(2) There are defects in the record design, it is inconvenient to fill in, and duplicate items are not merged.
(3) Write records in advance and make up records afterwards.
(4) The way of filling in the records is not uniform, and it is not strictly reviewed before filing, and it is inconvenient to check after filing.
Examples Of GMP Inspection Defects: The inspection records are suspected of falsification, and the instrument usage log is untrue. The liquid chromatograms of the refined Guanxin tablet powder printed on the liquid workstation of the enterprise, the batch numbers of which are 20130301, 20140501, 20140801, 20140802, and 20150901, are highly consistent, which is suspected of a data integrity problem of multiple uses for one image.
Records are the evidence left by GMP enforcement. GMP requires records to be timely, accurate, true, complete and revised in accordance with regulations, which requires the guidance of enterprise employees through inspection and training. The design of the record should be concise and optimized while complying with regulatory requirements to avoid repeated filling. In order to avoid filling in too much textual content, you should use more solidified content and then check it. In addition, when filing records on a regular basis, audit and inspection are required to correct problems existing in daily work in a timely manner.
-
Correct Your Mistakes
(1) Errors in document execution and record filling occurred repeatedly, and rectification was not implemented.
(2) For long-standing problems, the root cause has not been found and solutions have been formulated.
(3) Inspection and supervision are not fully covered and effectively implemented.
(4) Managers do not really recognize the mistakes in the quality management process.
(5) There is no change control for the changes that occur, which increases the risk.
Example Of Defects In GMP Inspection: The required injection volume of Citicoline Sodium Injection (content determination) is 10 μL, and the actual operation injection volume is 20 μL. The injection volume of the phase chromatogram was manually modified to 10 μL, and the enterprise audit did not find this problem.
Some companies claim that their corporate management system is running well, with no deviations and no changes, and even some managers are more resistant to pointing out problems in their management responsibilities and cannot accept and correct them with a good attitude. This is extremely undesirable. For recurring high-risk deviations, it is necessary to organize technical personnel to discuss key issues, and take appropriate corrective and preventive measures to control them.
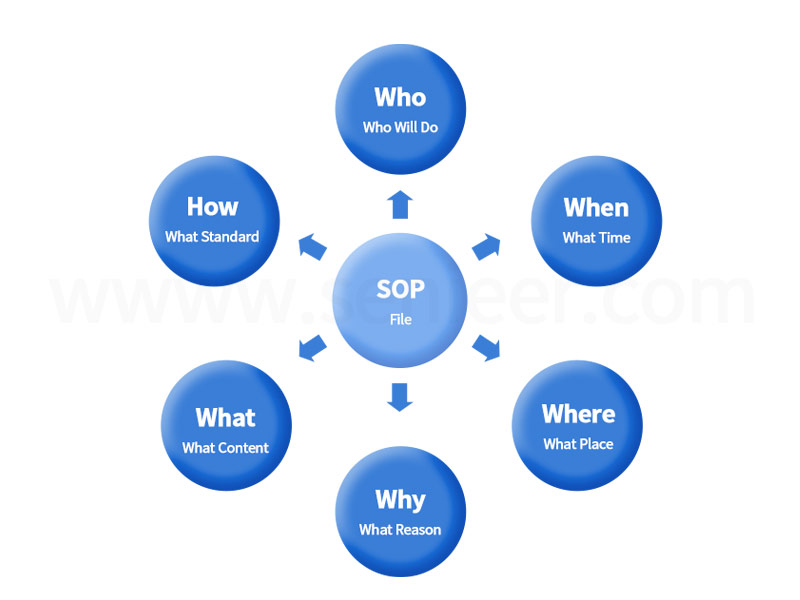
Figure 1 Requirements for writing SOP
Discussion On Improvement Methods
Each company has a different organizational structure and management process, but the essence of GMP management is the same. Personnel are the main body of the implementation of the combination of software and hardware, and the determinant of work quality. GMP implementation should first consider strengthening personnel and internal system management, and the improvement methods are suggested as follows:
-
The Decision-Making Level Establishes The Correct Orientation And Creates An Atmosphere Of Enterprise Self-Discipline
The decision-making level of the company is at the helm of the development of the enterprise, as well as the supervisor and inspector of the implementation of various measures. Article 4 of GMP (2010 Revised Edition) stipulates: Enterprises shall adhere to honesty and trustworthiness, and prohibit any false and deceptive behavior. This orientation must be correct, and pharmaceutical companies must strictly adhere to the bottom line of honesty and trustworthiness, and cannot break through at any time. The decision-making level of the enterprise must know the law, understand the law, and use it, and the consciousness and concept must be updated with the development of GMP, and create an atmosphere of strict self-discipline and compliance with laws and regulations in the enterprise, emphasizing certification over management, emphasizing hardware over software, and emphasizing benefits over talents. The idea of “Three Lights” must be changed [3], and the responsibility system should be implemented at the upper level of internal management, so that all kinds of limited resources can be reasonably allocated and applied. Managers at all levels cannot only report good news but not bad news. Managers should act like a funnel, not a filter. Only by transmitting information realistically can major risks be managed and controlled in a timely manner.
-
Pay Attention To The Effectiveness Of The Quality System And Attach Importance To The Construction Of Technical Teams
The 1998 version of GMP pays more attention to compliance and the 2010 version of GMP pays more attention to effectiveness. We should realize that compliance is not necessarily effective. In the process of implementing GMP, many pharmaceutical companies often have some documents required by laws and regulations, but they have not been applied in practice and have achieved actual results [4]. Documents are the code of conduct of the enterprise. First of all, it is necessary to establish a team with excellent technology and establish the document system in combination with the actual situation of the company. It is necessary to focus on the compilation principles such as content compliance, format and terminology uniformity, operability, consistency with reality, uniqueness of quotation, simple process and full participation. The compilation of the document system reflects the technical strength and management level of the technical team. It is also the basic guarantee of the effectiveness of the system, which is directly related to the quality of drugs.
In terms of technical team management, a system should be established to integrate organizational interests with personal interests. If the realization of organizational goals has little to do with personal interests, it is difficult to combine organizational goals with personal goals [5]. The management of core technical personnel is the key to enterprise management. It can attract and respect senior managers and give them higher welfare treatment. It can implement vocational and technical grade evaluation in the enterprise and establish a good team culture and lean management; Managers of different departments should carry out phased job change study, implement quality assessment system, strengthen the sense of responsibility and improve supervision and inspection; Different employees should have differentiated management, which should be linked with salary and welfare performance appraisal. Managers should not only have a good work plan, but also go deep into the front line for inspection and supervision, check the implementation of the plan and the implementation of document requirements, find out the actual problems and organize them to solve them in time.
-
Deep Understanding Of product Risk Management And Control, In-Depth Investigation And Scientific Decision-Making
Pharmaceutical companies conduct risk management and control over the entire process of product production, storage, transportation, and use, and conduct correct risk identification first, so as to determine correct risk control measures. It is necessary to find out high-risk items in a timely and comprehensive manner, implement key risk control and precise management for those items with high frequency, difficult to find, and serious consequences, analyze and study relevant data, and formulate corresponding CAPA measures.
Scientific use of quality tools provides guarantee for the effective implementation of GMP. Risk management should not be simply written using risk management tools to write a risk assessment report, but should be based on team experience and deep understanding and implementation of product quality and process. Evaluation Report. Risk assessment must be carried out before making a decision to make the decision more scientific, reasonable and operable.
-
Pay Attention To The Continuous Improvement Of Deviation Management And Encourage Continuous Innovation According To Local Conditions
Any deviation from procedures and standards should be included in deviation management. Deviation management is an important link in the implementation of GMP. As an effective means to find problems, analyze problems, solve problems and continuously improve the quality management system It is of great significance to improve the concept and improve the execution of quality improvement [6]. If you want to really improve the execution level of the enterprise, you must pay attention to every abnormality, form a multi-professional team to analyze carefully, constantly find the crux of the problem, and fundamentally solve the problem. This is the most effective way to improve the level of GMP management and execution.
According to the actual situation of the enterprise, continuous innovation of management methods can adapt to the continuous changes in the regulatory environment. For example, innovate inspection methods, implement unannounced inspections, surprise inspections, etc. within the enterprise, incorporate new inspection methods in inspections, and adopt horizontal pulling nets and vertical breakthrough inspection methods [7], so that GMP enters normal management; innovative training methods , make full use of network resources for mobile classroom training and assessment, achieve the purpose of systematically broadening knowledge, and enable outstanding technical personnel to conduct in-depth study and improve.
A valid implementation of GMP has no periods, only commas. Pharmaceutical companies should strive to improve execution efficiency and drug quality in light of the actual situation while meeting regulatory requirements. Pharmaceutical workers must always keep in mind the original intention of drug production quality management to treat diseases and save people, keep in mind the mission of serving the people wholeheartedly, and constantly explore and jointly promote the more compliant, orderly, stable and healthy development of the pharmaceutical industry.










