At present, due to the increasing variety of products of various pharmaceutical companies, the factors affecting the quality of drugs in the production process are also increasing. Therefore, it is necessary for pharmaceutical companies to establish a sound quality management system to systematically prevent quality problems and comprehensively improve the reliability of drugs.
Medicine is a special commodity, which is related to people’s life, health and safety. Customers have high requirements for its appearance and reliability. Therefore, in order to improve drug quality and control and reduce company management costs, it is absolutely necessary for pharmaceutical companies to establish a quality management system. By establishing a quality management system, pharmaceutical companies can analyze the cost of drug quality, which helps to promote the implementation of quality improvement plans; and through quality improvement, product reliability can be further improved and potential non-conformance problems can be prevented. In addition, this system can also help the management to grasp the problems existing in quality management; through the calculation and analysis of quality cost, the management of the enterprise can see the proportion of various management expenses, and have a specific understanding of product quality and the problems existing in quality management as well as the impact on the economic benefits of enterprises. The management can use this to make correct decisions on the quality management of the enterprise, which in turn will help the implementation of the quality promotion plan, provide sufficient resources for the construction of the quality management system, and form a virtuous circle.
The following will describe what needs to be done to establish a quality management system in a pharmaceutical company.
01 Quality System Culture Construction
By carrying out the construction of the quality system culture, publicizing the cultural values of the quality system and influencing the employees from the subconscious, the quality awareness of the employees has been improved. The company is constantly introducing new employees every year. In the face of these new employees, the construction of quality culture is very important. “It is much easier to cultivate a good habit than to correct a bad habit.” It is necessary for employees to fundamentally change their bad behaviors and habits at work and prevent quality incidents from happening.

According to the requirements of the State Administration of Market Supervision to regularly carry out the theme activities of “National Quality Culture Month”, companies should strengthen their internal quality month theme promotion, create a sense of responsibility to promote the establishment of “quality first”, and let employees in the quality promotion and culture month activities actively participate in the “Quality Management Improvement” activity, establish the awareness that everyone is a quality steward, create a good cultural atmosphere where all employees pay attention to quality, strengthen the overall quality management of the enterprise, promote the internal quality improvement of the enterprise, and enhance the quality competitiveness of the enterprise.
02 Establishment Of Quality System Documents
Establish enterprise standardization system documents, formulate quality policies and objectives, and standardize the company’s various work through the implementation of documents; ensure that each department can combine the company’s actual situation when compiling system documents to ensure the operability and adaptability of system documents; Regularly carry out internal audit and management review of the company’s quality system, judge the compliance and effectiveness of the quality system, continue to improve, continuously optimize the quality management process, and finally realize the high efficiency, standardization and standardization of quality management.
03 Establishment Of Production Process Management System
Because there are many factors affecting the production process, it is necessary to establish the control points of production risk factors and carry out risk assessment. Before production, provide guidance and training to production personnel to reduce personnel risks. During production, ensure that all production personnel can operate correctly in accordance with the SOP (standard operating procedure), double review, and avoid mistakes; make employees remember that in the event of sudden power outage, steam outage, water outage, air conditioner outage, etc. during the production process, they need to immediately stop production and report to the superior immediately, and continue production only after approval by the quality management department. After the production is over, the production personnel should first clear the site, remove items unrelated to production from the site, clean the room and equipment in accordance with the cleaning standard operating procedures, and make the cleaning status and cleaning validity period so that all the factors affecting product quality are within the controllable range. At the same time, to control each element of production, it is also necessary to strengthen the cooperation between various functional departments, solve problems in each process, link, communication and coordination process, give full play to the functions of each department, and escort production.
04 Establishment Of Quality Risk System
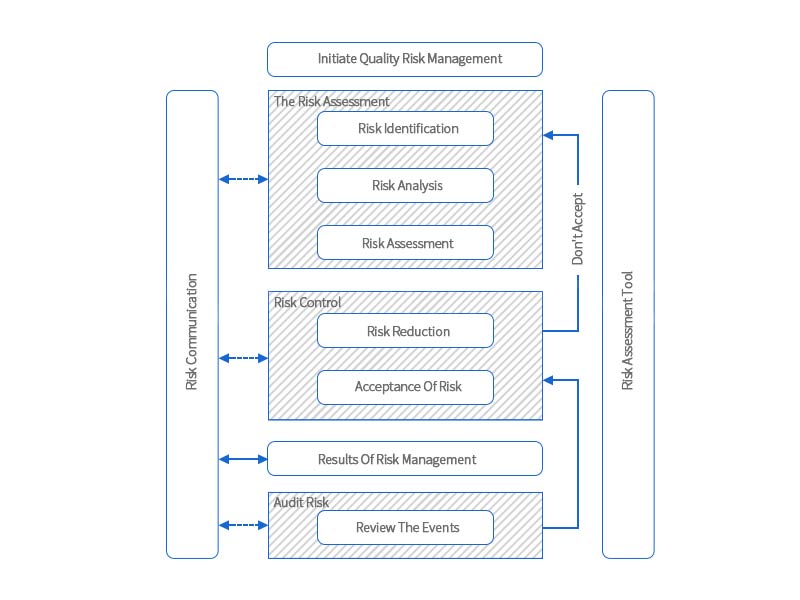
Figure 1 Quality Risk Management Flow Chart
In accordance with the principles of risk management in the Drug Administration Law of People’s Republic of China, we will keep the bottom line of drug quality and safety. First, collect information and materials related to risk assessment, organize analysis and evaluation of information and materials and make conclusions, establish a risk assessment team, determine the person in charge and necessary resources, and determine the working time limit for risk management procedures; secondly, carry out risk identification, risk analysis and risk evaluation, using different analysis tools for risk assessment for different risk projects, such as flowcharts, statistical analysis diagrams, cause-and-effect diagrams, fishbone diagrams, etc.; finally, formulate risk management and control measures to ensure the quality and safety of the company’s production of drugs, and the specific process of quality risk management (as shown in Figure 1). Risk assessment should also be actively carried out in the production process: regularly review suppliers, etc., and conduct quality control from the source; take effective risk control and preventive measures for identified risks in a timely manner, and carry out risk assessment and control. In addition, it is also necessary to formulate a post-marketing drug risk management plan in advance and actively conduct post-marketing research.
05 Establishment Of Supplier Quality Management System
It is also important to establish procedures for approval and management of suppliers of APIs, excipients, packaging materials, and other auxiliary materials. Before selecting a supplier, priority should be given to the supplier’s reputation, the supplier’s production capacity, and the supplier’s production cycle. When signing a purchase contract with a supplier, the supplier is required to provide: quality system certificate and statement, three recent batches Inspection report, chemical safety technical specification, quality standard, registration approval number and change document registration number, business license, production license, etc. that meet the company’s quality standards. The specific process of supplier confirmation (as shown in Figure 2). According to the supplier’s supply situation, the supplier’s supply situation and supplier qualification certificate and other materials should also be made into the supplier’s quality file, which should be updated regularly.
06 Establishment Of Laboratory Quality Management System
The establishment of laboratory safety and workflow management procedures is also related to the high-quality research and development of drugs. Laboratory work areas should be kept clean, free of clutter and obstructions. After completing any operation or every day’s work, it should be cleaned up in time; the experimenter should check the water, electricity, windows, doors, etc. before leaving get off work every day to ensure safety.
Before starting a new operation, identify potential physical, chemical or biological hazards and master proper safety precautions. Experimenters should fully understand the location, purpose and operation of emergency equipment in the laboratory, as well as the correct emergency procedures; when sampling, we should standardize the sampling behavior of raw and auxiliary materials and packaging materials to ensure that the samples taken are representative and uniform. Quality workers should establish management procedures for sampling rooms and cleaning procedures for sampling to avoid contamination of materials to be sampled; at the same time, establish procedures for the management of retained samples for inspection to ensure that the retained samples are representative and that their management and use conform to specifications.

In addition, the management procedures for the stability inspection of laboratory products and the management procedures for the transfer of analytical methods should also be established at the first time. In operation, we should also standardize the preparation, calibration and management of the titrant to ensure the accuracy and correctness of the preparation and calibration of the titrant, so as to ensure that the titrant used meets the requirements of the Pharmacopoeia and the current GMP. In addition, we can also standardize the management of standards and reference substances, standardize the management of all bacterial species in the microbiology laboratory, and standardize the use and maintenance of chromatographic columns to ensure the accuracy and reliability of the final test data. Experiment records and data processing are true and compliant.
07 Establishment Of Leadership Quality Awareness Improvement System
One of the main tasks of leadership is to manage people well, and managing people is mainly about managing people’s consciousness, mentality, ability and knowledge. Managing people requires someone with a certain level of authority to do it. Managers of many companies have heard of the herd effect: wherever the leading sheep goes, the following sheep follow, and the entire herd will continue to imitate the leader’s move. With a move, where the leading sheep go to “graze”, other sheep also go to “pan for gold”. As the saying goes: “The top is one foot, the bottom is one foot.” As the “leader” of the unit, the leader’s quality, work style, and personal image are often related to the development of a department or a unit. Once there is a problem with the “leader”, the direction of the entire flock is easy to deviate. Therefore, it is not only the boss’s business to manage people and lead the team well, but also the responsibility of the heads of various departments.
The company’s top leaders should draw a clear blueprint for the company’s future, formulate challenging strategic goals, and empower personnel at all levels with responsibilities and authorities, provide employees with the resources they need to perform their responsibilities, and motivate employees to achieve goals and continuous improvement. Contribution, top management shall demonstrate its leadership and commitment to the quality management system: take responsibility for the effectiveness of the quality management system, formulate the quality policy of the quality management system, and ensure that it is consistent with the organizational environment and strategic direction to ensure that quality integrate management system requirements into the organization’s business processes; promote the use of process methods and risk-based thinking in the business; ensure timely access to the resources required by the quality management system; keep in mind the importance of communicating effective quality management methods and implementing quality management system requirements. At work, motivate, guide and support employees in their efforts to improve quality, continuously drive improvement, support other managers in performing their responsibilities in related areas, and ultimately ensure that the expected results of the quality management system are achieved.
In today’s economy and trade, more and more pharmaceutical companies are rising rapidly, and the market competition is becoming increasingly fierce. Superior quality is a key to stand out, so companies must recognize the importance of quality management. Pharmaceutical companies apply systematic quality management systems to make production personnel in the production process operate in accordance with standard operating procedures to ensure that the obtained products meet quality standards and meet the licensing requirements for drug production and marketing.










