Dynamic Vapor Sorption Instrument (DVS) is a gravimetric vapor adsorption instrument. It uses a microbalance (accuracy can reach one millionth) to measure the weight change of the sample before and after adsorption and desorption under a certain relative partial pressure to determine the water absorption of the sample. The amount of adsorption and desorption of vapor or organic vapor.
DVS is a very important analysis method for food, medicine, daily necessities, textiles, polymer materials and other related industries. Almost all materials interact more or less with water vapor in the surrounding air. The change of material properties caused by moisture is a key factor affecting product production, packaging, storage, shelf life, etc.
The following introduces the specific application of DVS in pharmaceutical research and development:
1 Investigation On The Hygroscopicity Of Drugs
Hygroscopicity refers to the characteristic of the ability or degree of moisture absorption of the substance under certain temperature and humidity conditions. The test product is a solid drug substance that meets the quality standards, and the test results can be used as a reference for selecting appropriate drug packaging and storage conditions. The traditional method is to place the sample to be tested above the saturated salt solution with different concentrations, so as to study the hygroscopicity of the sample under different humidity. This method has a large sample volume, cumbersome operation, long test time, and the results are greatly affected by the external environment.
However, DVS determination only needs about 10mg to analyze accurately, with simple operation, short test time and stable test area. The figure below shows the DVS adsorption curve of sample B in the range of RH20%~80%:
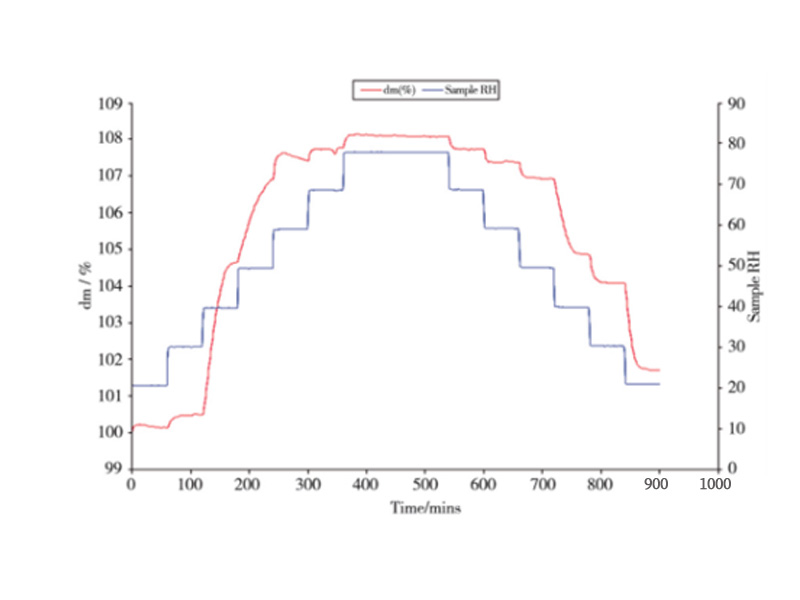
Fig.1 Water adsorption kinetic curve of sample B
When the relative humidity was 20%, the mass of sample B hardly changed; when the humidity was greater than 35%, the moisture adsorption increased significantly, and when the relative humidity gradually increased to 80%, the weight increased by 8% in 9 h. According to the guiding principles of the drug hygroscopicity test, the weight gain of the hygroscopicity is less than 15% but not less than 2%, which is hygroscopic, so sample B has hygroscopicity.
2 Comparing The Stability Of Different Crystal Forms Of Drugs
The same drug may exist in multiple crystal forms, and will change from one crystal form state to another with changes in environmental conditions (temperature, humidity, light, pressure, etc.), resulting in changes in the properties of the drug and affecting the quality of the drug. Therefore, it is necessary to study the stability of the crystalline substance state. DVS is also a common method to investigate the stability of different crystal forms of drugs. The following are the DVS adsorption-desorption curves of each crystal form of sample C:
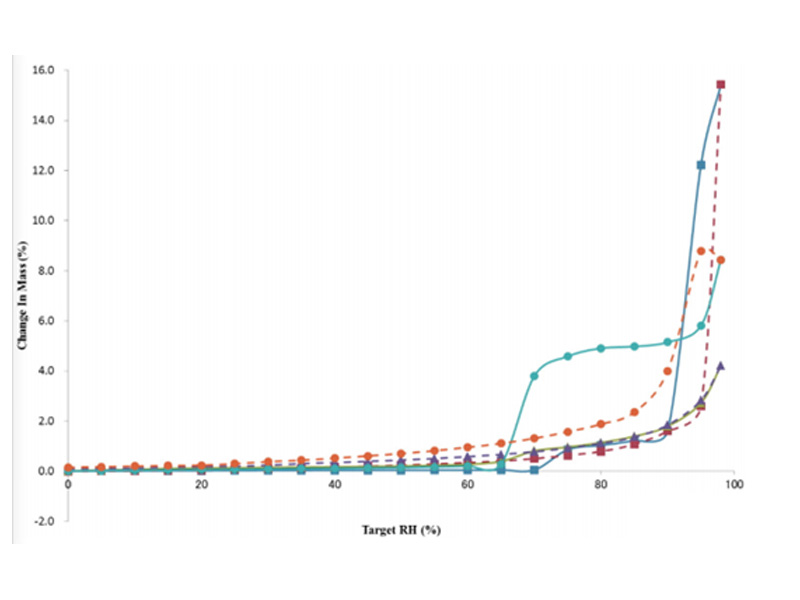
Fig. 2 Adsorption and desorption curves of different crystal forms of sample C. Type I of C (square), Type II of C (triangle), Type III of C (circle). The solid and dashed lines are the adsorption and desorption curves, respectively.
From the figure above, it can be seen that during the adsorption process, the mass of type III increased by about 5% at 75% relative humidity, and all C crystals showed deliquescence above 90% RH. During desorption, the curve of type II and the adsorption curve almost completely overlap, indicating that the structure has no change before and after; while type III and type I do not desorb according to their own adsorption curves, but are close to the change curve of type II, and when adsorbed Both have a step change, and it is speculated that there is a transformation between their structures. Therefore, it can be preliminarily judged that the stability order of C crystal is type II > type I > type III.
3 Phase Transition Of Steam-Induced Hydrates
Steam-Induced Hydrate and Phase Transition Studies Adsorption, desorption and re-adsorption, desorption in an isothermal, increasing humidity environment. The process of water vapor adsorption and desorption of drugs is often accompanied by phase transition or hydrate transition. According to the moisture adsorption isotherm curve in DVS, the stoichiometry of the structure is calculated, and then the transition kinetic process is judged. The figure below shows the DVS curve of theophylline.
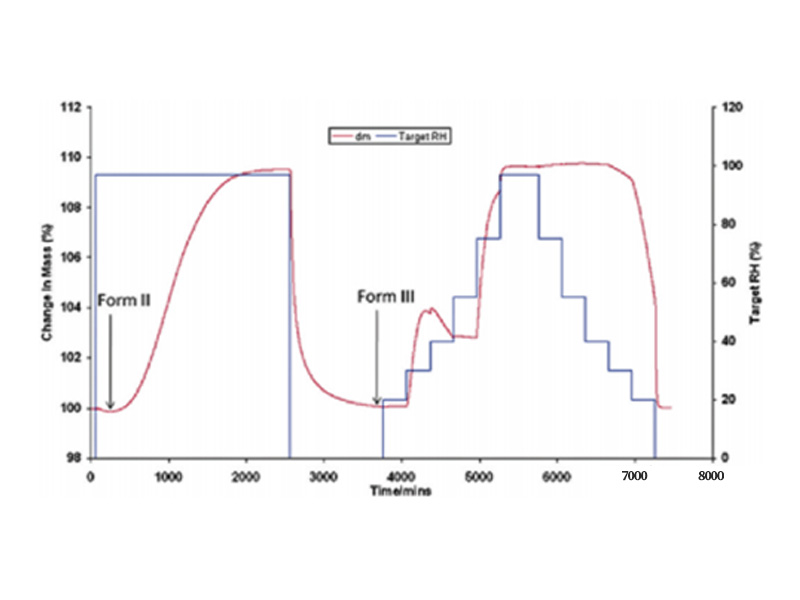
Figure 3 Adsorption And Desorption Of Type II Theophylline
It can be seen from the figure above that the anhydrous II phase is adsorbed and transformed into a monohydrate at a humidity of about 97%, and the monohydrate is transformed into a III phase during the desorption process of the first cycle. While in the second round of adsorption process, in the range of 30-50% humidity, the curve is hump-like – as the humidity increases, the quality shows a downward trend, which is due to the transition from III to II phase.
4 Judgment Of Amorphous Content
Amorphous form affects drug efficacy, storage and stability, etc., so accurate determination of amorphous content is more important. Common PRXD determination of amorphous content requires 300-400 mg of sample, and the LOD can reach 10%; DSC detection requires 4-10 mg of sample, and the LOD reaches 5%; while DVS measures 5-50 mg of sample, the LOD can reach 0.05%
The figure below shows the DVS curve of a micronized sample:
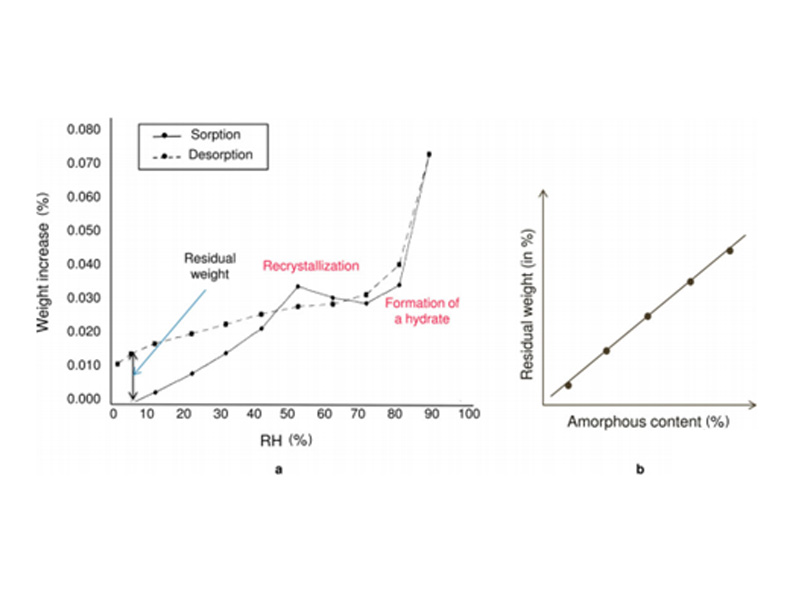
Fig. 4 a graph – the adsorption-desorption curve of a micronized sample, b graph – the standard curve of residual weight and amorphous content
It can be seen from Figure a that when RH increases from 5% to 95%, the sample absorbs water and is difficult to detect; but when crystallization occurs, the sample will release water with the formation of monohydrate and become hydrophilic Then lower. Comparing the spectra of adsorption and desorption, the amorphous content in the sample was calculated by the function of residual weight versus water absorption in Figure b.










