Purified water systems are an important part of pharmaceutical production. From the perspective of “quality comes from design”, this paper will introduce the composition of purified water systems, propose the potential risks that need to be paid attention to in the design of purified water systems, and give corresponding solutions to ensure the stable and reliable quality of pharmaceutical water.
Purified water is a very critical excipient that can be used both as a process feedstock (i.e. for pharmaceutical production) and as a utensil for cleaning. Therefore, the pharmacopoeias of various countries have made strict provisions on the quality standards of pharmaceutical water. This article will discuss the key points of selecting a purified water system from the perspective of pharmaceutical water use, and how to ensure that the purified water system is stable to produce purified water that meets the requirements of the standard.
1 Definition, Scope Of Application And Quality Standards Of Purified Water
There is a definition and scope of application of purified water, which can be found in the appendix of the Pharmacopoeia of the People’s Republic of China (2010 Edition). Purified water definition: Purified water is pharmaceutical water prepared by distillation, ion exchange, reverse osmosis or other suitable methods for drinking water. It does not contain any additives and the quality should comply with the provisions of the Purified Water Standard. The standards currently used internationally for quality standards for purified water vary, as detailed in Table 1 [1].
2 Critical Control Points For Purified Water Systems
Purified water system mainly includes pretreatment system, water production system, storage and distribution system, etc.; The design phase is critical to ensuring that the quality of the final purified water is stable and reliable, determining whether the quality of the resulting water meets the standard requirements.
Water production system is usually divided into: pretreatment + primary reverse osmosis + secondary reverse osmosis; Primary reverse osmosis + secondary reverse osmosis + EDI; Pretreatment + ion exchange method. When choosing a water production system, enterprises should focus on the stability of the water quality and the economy of the equipment from the two aspects of the system. Taking the purified water system installed by Lianyungang Kangle Pharmaceutical Co., Ltd. as an example, the attention points of the new purified water system are as described below (the purified water production process is shown in Figure 1).
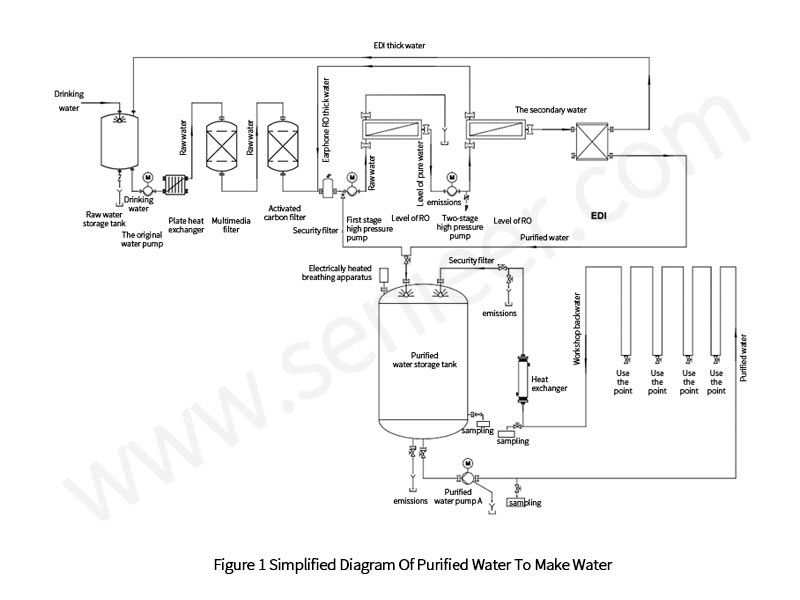
2.1 Pre-Processing
Mechanical filtration is the most commonly used method in the pretreatment process. However, with the advent of ultrafiltration devices, mechanical filtration has been replaced. If mechanical filtration is used for pretreatment, sand filters, activated carbon filters and resin softeners are essential as a combination of processes.
In daily use, alum and particulate matter are produced when adding medicinal agents, which are very easy to accumulate in sand filters and activated carbon filters, and it is difficult to completely remove them even if they are backwashed. While causing the passage to be blocked, it will also form a groove when making water, making the pretreatment process of raw water incomplete. In this regard, the sand filter filter and activated carbon filter can be blown back through compressed air, and the quartz sand and activated carbon deposited in the filter are blown away before the filter is washed and backwashed, thereby greatly improving the positive washing and backwashing effect.
In the process of operation, the organic matter adsorbed by the sand filter filter and the activated carbon filter is easy to cause the breeding and reproduction of microorganisms, resulting in excessive microbial content in the effluent. This phenomenon can be avoided by using appropriate disinfection methods (e.g. ozone disinfection, pasteurization) and the activated carbon filter needs to be disinfected regularly. Enterprises located in the north, due to the low water temperature in winter, it is recommended to use pasteurization method, plate heaters are installed after the original water pump, which can be used as pasteurizers during disinfection and used as raw water preheaters during winter production.
For resin softeners, due to the large amount of salt required for resin regeneration, frequent regeneration cleaning operations are not only time-consuming and labor-intensive, but also cause corrosion to stainless steel by water-soluble chloride ions. In 2014, he participated in the construction of a new 12 t/h purified water system, when, after several analyses of the raw water with the equipment manufacturer, it was decided to cancel the resin softener and replace it with a primary RO system, which can realize both desalination operation of the pretreatment process and can also be used as AN RO Membranes for water production systems. Since its installation and use, not only has the treatment effect been in the expected value, but also greatly saving salt and manpower.
Compared with the commonly used pretreatment methods, the advantages of ultrafiltration devices in the pretreatment of raw water are obvious – there is no need to add flocculants and coagulants, avoiding the subsequent complicated removal process. The ultrafiltration device can also remove some microorganisms and organic matter, so that not only the quality of the effluent water is effectively guaranteed, but also the service life of the RO membrane is extended.
2.2 Water Production Systems
For the water production system, Lianyungang Kangle Pharmaceutical Co., Ltd., where it is located, adopts the method of primary RO + secondary RO + EDI, and the purified water quality produced therefrom is stable and reliable. In the construction of the water system, how to do a good job of communication with the equipment manufacturers in the early stage is the key, brainstorming in the exchange, in order to improve the design scheme, can effectively solve the pain points in the water production process. The following is a summary of the experience of the construction of the water system according to the company.
The system eliminates the intermediate water tank after the water production system first-level RO membrane pure water. Primary RO membrane pure water will enter directly from the secondary RO membrane high-pressure pump, thereby reducing the risk of contamination and microbial growth during the water making process.
After the raw water undergoes primary and secondary reverse osmosis, the conductivity will also decrease. The secondary RO concentrated water is connected to the water inlet of the primary RO high-pressure pump, and the EDI concentrated water is sent to the raw water storage tank, which will not only increase the water production rate, but also save the waste of water.
During system production, the conductivity increases due to the introduction of CO2 from the air. At present, the common practice of enterprises is to add NaOH before the secondary RO enters the water, and remove the CO2 in the water by adjusting the pH value. But in numerous audits of EU clients, several auditors have argued that this approach needs to be justified as to why NaOH is added as an additive and not as another substance. Therefore, enterprises may consider the use of degassing devices when formulating user requirements statements (URS).

The membrane degassing device is covered with a hollow fiber membrane, and because the membrane is hydrophobic (water cannot pass through, but air can pass), when pure water passes through the membrane degassing device, the CO2 gas is sucked away by vacuum. No chemical reagents are added to remove CO2 from pure water.
The control of microorganisms in the water production system should start from two aspects. On the one hand, it should be realized from the design to ensure the cleanliness of the environment so that microorganisms cannot breed. For example, the pure aquatic water system implements 24 h non-stop work, using a self-controlled interlocking method: when the system produces water, the system automatically produces at full frequency; When the water level of the purified water storage tank of the distribution system reaches a high water level, the system automatically reduces the frequency and turns off the water inlet of the purified water storage tank, which is instead fed into the first stage RO booster pump. The advantage of microbial control in this way is that it maintains the flow of water in the pipeline and membrane system, avoiding the generation of stagnant water and breeding microorganisms. On the other hand, regular disinfection can avoid the proliferation of microorganisms. For example, the membrane system is disinfected regularly, chemical disinfection and pasteurization are the most commonly used disinfection methods. The pasteurization method requires the replacement of primary RO, secondary RO, and EDI membranes with high-temperature membranes at the URS stage. The law of chemical disinfection is to disinfect the membrane system with chemical reagents, common reagents peroxide disinfectants, phenolic disinfectants, chlorine disinfectants, etc., when choosing, it is necessary to avoid damage to the membrane.
2.3 Storage And Distribution Systems
Storage and distribution systems [1,2] mainly include storage, distribution, and water points. The quality of the water in the production process is stable and reliable, the amount of water used at each water point matches the pressure, and the reasonable investment and operating costs are the keys to measuring a good storage and distribution system. For water equipment manufacturers, this is extremely challenging. There are currently a variety of storage and distribution systems, the more common ones are in-line and parallel, as shown in Figures 3 and 4.
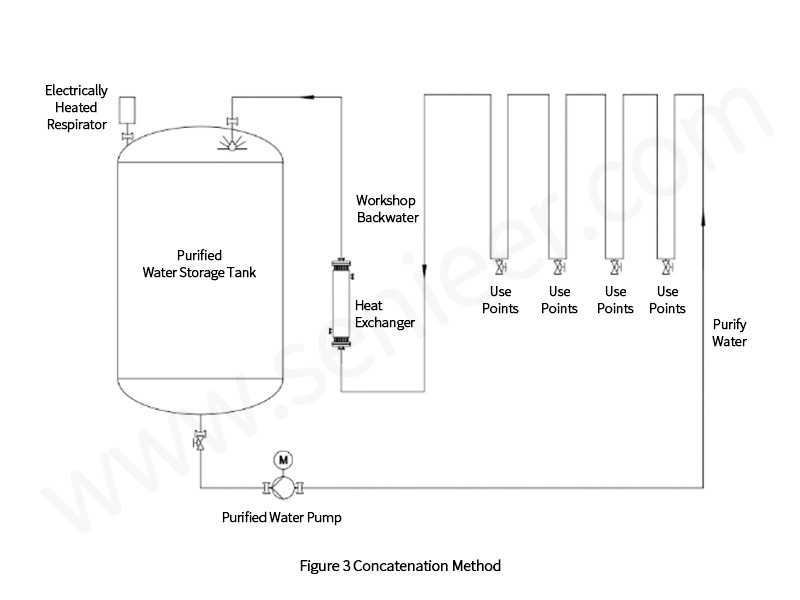
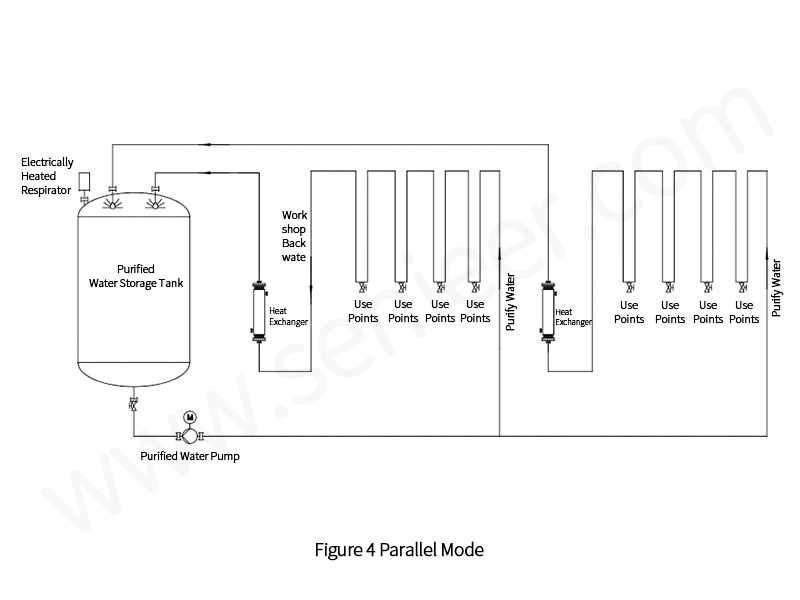
A good storage and distribution system is crucial to the stability and reliability of pharmaceutical water, and the following experiences are summarized.
The storage part is mainly composed of a tank system. Common storage tanks are divided into vertical storage tanks and horizontal storage tanks, because the effectiveness of horizontal storage tank cleaning and disinfection is easy to be restricted, so priority should be given to vertical storage tanks. Its advantages are as follows: the sewage outlet of the vertical storage tank is generally located at the bottom of the tank, which is easy to drain the water in the system.
The upper and lower limit points of vertical storage tanks are equally spaced, and the water storage is more, and the “residual” water below the lower limit point is less.
When it comes to spray cleaning, vertical tanks are simpler.
Regarding the design of storage and distribution systems, there are the following comments:
The size of the tank is a very critical parameter. From the perspective of quality control, the smaller the volume of the tank, the better, because maintaining a high turnover number (the number of times the water in the tank is changed within 1 h) can significantly reduce the reproductive rate of microorganisms; From an engineering point of view, the use of a small volume tank system is not feasible, can not meet the production at the peak of the flow of water supply. Considering the turnaround time, it is recommended that the number of turnovers be 2 to 3 times.
During the cleaning and disinfection process, the inability to exhaust itself is another factor that causes microbial contamination. In order to avoid this situation, the pipeline needs to maintain a certain slope, which is conducive to draining the liquid in the pipeline under the action of gravity. Currently, according to the ASME BPE standard, the slope of purified water should not be less than 1% of the horizontal benchmark.
The existence of dead angles will form stagnant water areas in the system, and the fluidity of the water body is very poor, which can easily lead to a large number of microorganisms breeding and forming a microbial film, causing microorganisms, total organic carbon (TOC) and other indicators to exceed the standard. It is recommended to follow the “3D” principle instead of the “6D” principle [1].
The water consumption and instantaneous maximum flow rate of the workshop use point should be reasonably counted. Through calculations, the diameter of the conveying pipe and the pump are selectively installed to ensure the flow rate of purified water in the pipeline. The flow rate is too low, which is easy to cause the breeding of microorganisms, and the International Association of Pharmaceutical Engineering (ISPE) recommends that the return water flow rate should not be less than 0.9 m/s.
The quality of the water in the system is also affected by the way and when it is disinfected. Common disinfection methods are: chemical disinfection (such as the use of hydrogen peroxide), pasteurization, ozone disinfection and so on. The disadvantages of chemical disinfection are obvious, that is, the residual problem is difficult to solve; For ozone disinfection, the ozone concentration is unstable, easy to decay and easy to react with toc in water, etc., which are all subtractions from this disinfection method; Although the method of returning water UV (ultraviolet light) can decompose the ozone in it, the problem of ozone residue is also inevitable. Compared with the previous two methods, the advantage of pasteurization is that only by heat disinfection (the temperature is controlled above 80 ° C, hot water circulation disinfection for 2 h), microorganisms can be controlled at a low level (less than 50 CFU/ml) without considering the audit problems. Combining several disinfection methods, it is recommended that the pasteurization method be preferred[2].
3 Conclusions
When selecting a purified water system, companies should start from the perspective of pharmaceutical GMP and consider how to control pollution and prevent microbial growth from the design stage. In this paper, some insights are mainly put forward from the experience of the use of purified water and the design and installation, hoping to provide a useful reference for pharmaceutical companies to improve the quality of pharmaceutical purified water systems.










