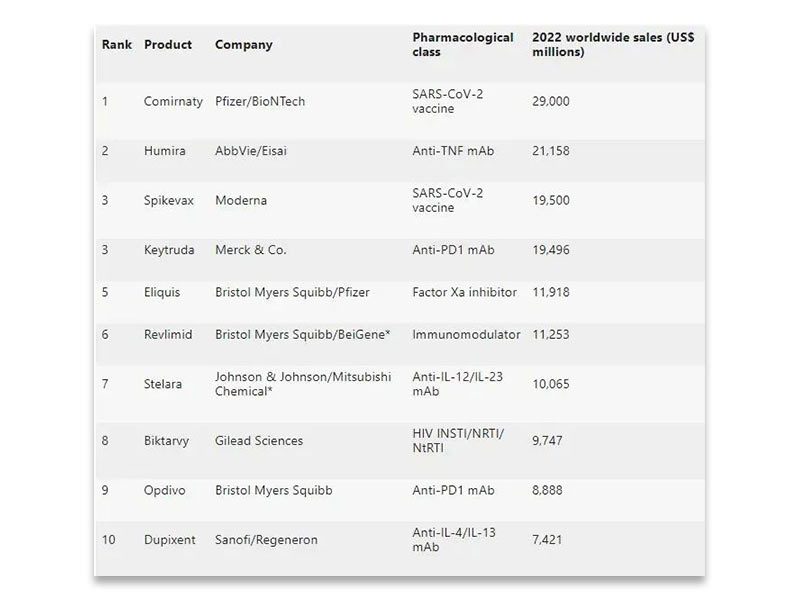
Recently, Nature’s sub-journal “Nature Review Drug Discovery” and biopharmaceutical industry media Evaluate Vantage both published articles to analyze and predict the top 10 global drug sales in 2022. Coincidentally, two analysis articles gave the same prediction. In the list, two products are predicted to have sales of more than 20 billion US dollars, with the top-selling product at 29 billion US dollars (approximately RMB 184.4 billion), and there are additional 5 products whose sales are predicted to exceed 10 billion US dollars. Bristol-Myers Squibb may be the biggest winner, involving three drugs.
1. Comirnaty
| Name | Comirnaty
|
| Drug Type | Vaccine |
| Company | Pfizer/BioNTech
|
| Estimated Sales in 2022 | $29 billion |
As the first mRNA COVID-19 vaccine approved by the regulatory authorities, Comirnaty has become the backbone of Pfizer’s rapid growth in performance. According to Pfizer’s 2021Q3 financial report data, in this single quarter alone, Comirnaty achieved revenue of $12.977 billion. Since then, its cumulative sales revenue in the first three quarters has reached 24.277 billion US dollars.
The rapid market volume of Comirnaty is due to the continuous expansion of the vaccinated population. So far, Comirnaty has been approved by Emergency Use Authorization (EUA) for the prevention of COVID-19 in adults over 16 years old, people aged 12-15, and children aged 5-11. At the same time, the vaccine was also granted an EUA as a single-dose booster shot for people 65 years of age and older, 18-64 years old at high risk for severe COVID-19, 18-64 years old with frequent institutional or occupational exposure to SARS-CoV-2, individuals who have completed primary immunization with a different COVID-19 vaccine. Currently, Comirnaty is still conducting clinical trials in children between 2 years to under 5 years old and 6 months to under 2 years old.
In May 2021, BioNtech and Fosun Pharma announced the establishment of a joint venture for the localized production and commercialization of mRNA COVID-19 vaccine products, with an annual production capacity of 1 billion doses, but so far, this vaccine has not been approved in China.
2. Humira
| Name | Humira
|
| Drug Type | Fully Human Anti-Tumor Necrosis Factor Monoclonal Antibody |
| Company | AbbVie
|
| Estimated Sales in 2022 | $20.4 billion |
Humira (trade name: Humira) is the world’s first fully human anti-tumor necrosis factor monoclonal antibody approved for marketing. It was first approved by the US FDA in December 2002 for the treatment of moderate to severe rheumatoid arthritis. Approved by the European EMA in September 2003, it has been approved globally for more than 15 indications including rheumatoid arthritis, ankylosing spondylitis, psoriasis, and pyoderma gangrenosum.
Known as the “King of Medicines”, Humira is sold in more than 96 countries or regions, ranking first in the world’s best-selling drug list for many consecutive years. Since its launch, its cumulative sales have exceeded 170 billion US dollars. The revenue in the first three quarters of 2021 was 15.36 billion US dollars, and as the COVID-19 epidemic continued, it fell from the top 1 champion of the best-selling drug which it has won for many times.
At the same time, a more serious problem for Humira is the imminent patent expiration, and the company has been struggling to defend its patent rights based on the huge annual revenue Humira brings to AbbVie. Since AbbVie signed the first Humira biosimilar settlement agreement with Amgen in 2017, the company has subsequently signed 8 similar agreements, including with Boehringer Ingelheim, Pfizer, Mylan, Sandoz, etc. Its patents in the U.S. market basically stop in 2023, and it is expected that at least 9 biosimilars will enter by then, dividing up Humira’s market share.
3. Spikevax
| Name | Spikevax
|
| Drug Type | Vaccine |
| Company | Moderna
|
| Estimated Sales in 2022 | $19.5 billion |
As an mRNA COVID-19 vaccine, Spikevax also occupies a high position in the list. It was developed by Moderna and has been approved by the European Medicines Agency (EMA) and Health Canada, and it is expected to receive full FDA approval by April 2022.
4. Keytruda
| Name | Keytruda
|
| Drug Type | PD-1 monoclonal antibody |
| Company | Merck & Co.
|
| Estimated Sales in 2022 | $19.5 billion |
Previously, Keytruda has been highly expected to surpass the “King of Medicine” Humira. Its revenue in 2021 is 14.38 billion US dollars, which has ranked in the top 2 of the world’s best-selling drugs in 2020, and its revenue in the first three quarters of 2021 is 12.578 billion US dollars.
In the past few years, Keytruda has been approved for new indications almost every year. Currently, more than 30 indications have been approved globally, including melanoma, non-small cell lung cancer, small cell lung cancer, head and neck cancer, and Hodgkin lymphoma. tumor, large B-cell lymphoma, bladder cancer, colorectal cancer, gastric cancer, esophageal cancer, cervical cancer, liver cancer, Merkel cell cancer, kidney cancer, endometrial cancer, etc. At the same time, Keytruda is still conducting more than 400 clinical trials research projects, involving more than 30 types. In the future, the market potential will be further expanded.
5. Eliquis
| Name | Eliquis
|
| Drug Type | small molecule drug |
| Company | Bristol-Myers Squibb/Pfizer
|
| Estimated Sales in 2022 | $11.9 billion |
Eliquis is a coagulation factor inhibitor jointly developed by BMS and Pfizer. The drug was initially approved for marketing in the EU in May 2011. It is mainly used for the prevention of venous thromboembolism in patients undergoing hip or knee replacement surgery. In 2012, Eliquis was approved by the FDA to reduce the risk of non-valvular atrial fibrillation (AF) in patients with stroke and systemic embolism, making it the third globally marketed new-generation oral anticoagulant.
Thanks to the huge market demand for anticoagulants and the superiority of Eliquis itself, Eliquis can significantly reduce the risk of major bleeding. Eliquis has become the world’s best-selling anticoagulant drug, with global revenue of 8.088 billion US dollars in the first three quarters of 2021.
6. Revlimid
| Name | Revlimid
|
| Drug Type | small molecule drug |
| Company | Bristol-Myers Squibb
|
| Estimated Sales in 2022 | $11.2 billion |
Revlimid is also a super blockbuster drug. Bristol-Myers Squibb acquired Revlimid by completing the acquisition of Celgene at the end of 2019. The drug brought Bristol-Myers Squibb 12.15 billion US dollars in revenue in 2020, becoming the company’s drug that contributed the most to the oncology revenue segment that year, As a comparison, Bristol-Myers Squibb’s aother flagship drug, Opdivo, had sales of only $6.992 billion that year. Revlimid’s sales performance continues to refresh. In the first three quarters of 2021, Revlimid achieved a total revenue of 9.486 billion US dollars.
It is worth mentioning that the core patent of Revlimid (Composition of Matter) has expired in 2019, and most of the other patents will expire around March 2022. Four other generic companies have now been approved to sell a similar drug to the oncology drug Revlimid (lenalidomide) after March 2022.
7. Stelara
| Name | Stelara
|
| Drug Type | Monoclonal Antibody |
| Company | Johnson & Johnson
|
| Estimated Sales in 2022 | $10.1 billion |
With its unusual mechanism of action and the continuous expansion of approved indications, Stelara’s sales performance has increased frequently. Its sales in 2020 hit a new high of 7.707 billion US dollars, and the compound annual growth rate from 2010 to 2020 reached 34.7 %. It has undoubtedly played a mainstay role in Johnson & Johnson’s entire pharmaceutical business segment, with sales of 5.802 billion US dollars in the first three quarters of 2021.
However, Stelara is also facing the dilemma of patent expiration. According to public information, the main US patent of Stelara will expire in 2023, and the product will also face huge challenges with biosimilars. Several biosimilar companies are lining up, with South Korea’s Celltrion currently leading the pack with a Phase III product.
8. Biktarvy
| Name | Biktarvy
|
| Drug Type | compound preparation |
| Company | Gilead
|
| Estimated Sales in 2022 | $9.7 billion |
Biktarvy is an integrase strand transfer inhibitor consisting of bictegravir (50mg), emtricitabine (200mg) and tenofovir alafenamide (25mg). It was approved for marketing in February 2018 and is suitable for the treatment of HIV-1-infected children and adults weighing at least 25 kg as a complete regimen. Biktarvy was approved in China in August 2019 as a complete regimen for the treatment of HIV-1-infected adults who have no current and previous evidence of viral resistance to integrase inhibitors, emtricitabine or tenofovir. Biktarvy is expected to continue to dominate HIV treatment with its daily oral triple therapy, with sales of 6.094 billion US dollars in the first three quarters of 2021.
9. Opdivo
| Name | Opdivo
|
| Drug Type | PD-1 monoclonal antibody |
| Company | Bristol-Myers Squibb
|
| Estimated Sales in 2022 | $8.9 billion |
As the world’s first approved PD-1 inhibitor, since its approval in July 2014, Opdivo has been approved in more than 60 countries and regions for the treatment of more than ten types of cancer, including lung cancer, head and neck cancer, gastric cancer, and esophageal cancer. , liver cancer, kidney cancer, colorectal cancer, urothelial cancer, melanoma, Hodgkin lymphoma, pleural tumors, etc.
Opdivo was once regarded as Keytruda’s strongest competitor. At the beginning of the PD-1 drug sales, Opdivo’s sales were twice that of Keytruda, but in recent years, with the continuous expansion of Keytruda’s indications, Opdivo’s sales began to show weakness. In the first three quarters of 2021, its global sales were 6.03 billion US dollars, less than half of Keytruda’s.
10. Dupixent
| Name | Dupixent
|
| Drug Type | Fully Humanized Monoclonal Antibody |
| Company | Sanofi/Regeneron
|
| Estimated Sales in 2022 | $7.4 billion |
Dupixent is a fully humanized monoclonal antibody that inhibits the signaling of IL-4 and IL-13. It was approved by the FDA at the end of March 2017 and has been approved to treat a variety of diseases caused by type 2 inflammation: Moderate to severe atopic dermatitis (patients ≥6 years old), moderate to severe asthma (patients ≥12 years old), chronic rhinosinusitis with nasal polyps (CRSwNP, adult patients), etc.. Its sales in the first three quarters of 2021 amounted to 6.8 billion US dollars.
Some analysts predict that Dupixent is expected to surpass Biktarvy in 2026, becoming a member of the drugs with ten billion sales.